ADVANCED THERAPEUTICS
We aim to develop biocompatible polymer formulations structured in nanoparticles and hydrogels that can be used as advanced vehicles for therapeutics in stroke and neurodegeneration (Alzheimer's and Parkinson diseases). Our goal is to overcome actual limitations of classic systems of drug and stem cell-based delivery that until now have translated into poor clinical outcomes.
We also aim to develop strategies of stem cell-based therapeutics, focused in the use of intrinsic (stem cells) and extrinsic (microenvironment) preconditioning, as well as the optimization of new mobilization/homing paradigms, to stimulate host stem cell potential.
Our laboratory is seeking to develop a drug-release system based on silk fibroin. We have tested the biocompatibility of silk fibroin within the Central Nervous System (CNS), thus demonstrating the suitability of this biomaterial for our purpose. We are able to generate different formats with silk fibroin: hydrogels, films, nanoparticles, etc. We have shown the therapeutic possibilities of the encapsulation of stem cells inside hydrogels in stroke treatment. The basis of this therapeutic approach are the creation of a microenvironment that will protect the cells from an hostile environment, as it is the damage brain, while they act as growth factor factories. We also aim to prove the feasibility of delivering different drugs and biomolecules, alone or in combination, from silk fibroin formats to the damaged CNS.

Main routes to target the stroke brain with biomaterial-based nanoparticles and hydrogels. González-Nieto et al. 2020. Biomaterials to Neuroprotect the Stroke Brain: A Large Opportunity for Narrow Time Windows. doi: 10.3390/cells9051074.

Histological identification of silk fibroin deposits over time after intracerebral injection. These results were obtained in collaboration with Biomaterials and Regenerative Engineering laboratory from the Centre for Biomedical Technology. Fernández-García et al. 2016. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. doi: 10.1016/j.actbio.2016.09.003.
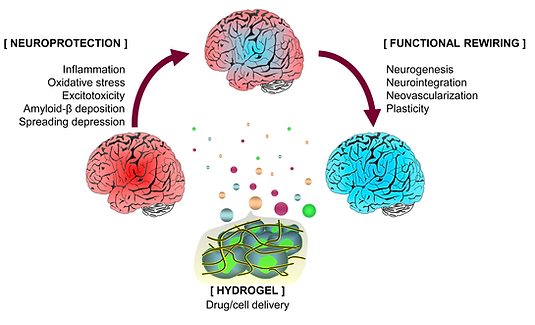
Hydrogel-based therapeutics sustains drug delivery and support cell survival and engraftment after implantation. Fernández-Serra et al. 2020. Hydrogels for neuroprotection and functional rewiring: a new era for brain engineering. doi: 10.4103/1673-5374.268891.

Representative images and quantification of mSCs expressing the EGF protein (EGFP) implanted into the striatum of non-EGFP-expressing mice over time. These results were obtained in collaboration with Biomaterials and Regenerative Engineering laboratory from the Centre for Biomedical Technology. Fernández-García et al. 2018. Cortical Reshaping and Functional Recovery Induced by Silk Fibroin Hydrogels-Encapsulated Stem Cells Implanted in Stroke Animals. doi: 10.3389/fncel.2018.00296.
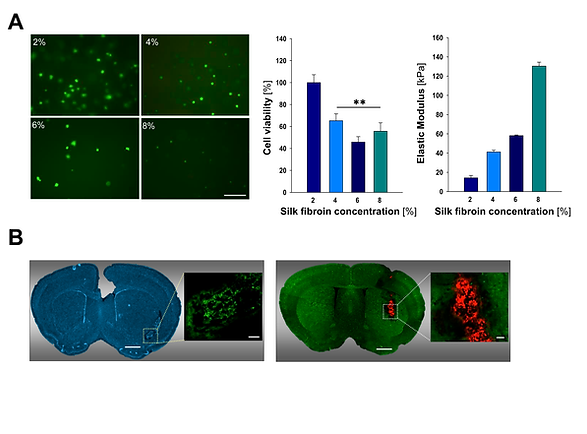
(A) Viability of mesenchymal stem cells encapsulated in silk fibroin hydrogels and stiffness properties of silk fibroin hydrogels. (B) Images of coronal brain sections showing the location of mesenchymal stem cell populations encapsulated into 2% silk fibroin hydrogels after implantation into the striatum of non-EGFP (left panel) and EGFP expressing mice (right panel). Fernández-Serra et al. 2020. Hydrogels for neuroprotection and functional rewiring: a new era for brain engineering. doi: 10.4103/1673-5374.268891.
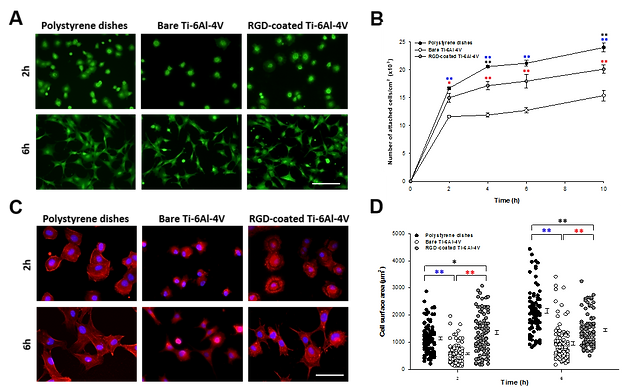
Functionalization strategies to enhance the biocompatibility of biomaterials for drug and cell delivery. In this study, titanium (Ti-6Al-4V)-based metallic surfaces were modified with the RGD peptide present in the extracellular matrix protein fibronectin.
Adhesion and spreading analysis of MSC seeded on polystyrene, bare Ti-6Al-4V and RGD-coated Ti-6Al-4V. (A) Representative fluorescence microscopy images of calcein positive stained MSC at 2 and 6 h after seeding (scale bar: 140 µm). (B) Number of attached cells/cm2 across the time after seeding. (C) Fluorescence microscopy images of Phalloidin/Hoechst stained MSC at 2 and 6 h after seeding (scale bars: 60 µm). (E) Averaged surface area of MSC on the different surfaces. A. Álvarez-López et al., Improved cell adhesion to activated vapor silanization-biofunctionalized Ti-6Al-4V surfaces with ECM-derived oligopeptides, Materials Science and Engineering C. (2021). doi: 10.1016/j.msec.2021.112614
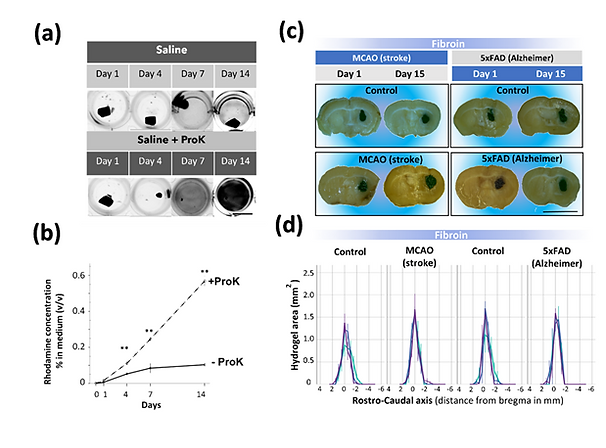
In this study, we analyzed the stability of silk fibroin hydrogels exposed to different in vitro and in vivo neuroinflammatory environments. (a) In vitro, silk fibroin is not degraded by activated microglia or enzymatic digestion with microglia-derived metalloproteinases. (b) However, the stability of SF was only compromised upon enzymatic incubation of the material with Proteinase K (Saline + ProK) causing the unbound of the fluorescent probe and detection of a fluorescent signal in the surrounding hydrogel conditional medium. (c) In vivo, SF hydrogels implanted in the brain were very stable and did not show signs of extensive degradation in extreme inflammatory models of stroke (MCAO model) and Alzheimer´s disease (5xFAD model), whereas similar amounts of collagen hydrogels rapidly disappeared after cerebral implantation. Yonesi et al. 2023. Resistance to Degradation of Silk Fibroin Hydrogels Exposed to Neuroinflammatory Environments. doi:10.3390/polym15112491
